Dr. Andrew S. Mount Uses Stella® to Research, Teach, and Test Product Ideas
 Andrew Mount
Andrew Mount
Andrew S. Mount, PhD, founder, ConusCoat LLC, has spent his career asking hard questions.
How do oyster shells regenerate? How fast will a virus spread and how many people will be
infected? Will marine environments improve if boat hulls are sealed with non-toxic paints?
Mount has asked, and answered, those questions as a master’s student at College of Charleston,
PhD candidate, research scientist, and college professor at Clemson University, and now as an
innovative product developer and founder of ConusCoat LLC. Through those endeavors, he has
used Stella to guide his and his students’ thinking to find and communicate new insights.
“Anyone can use Stella – you don’t have to be an engineer,” says Mount. “Stella’s graphical
interface makes it easy for anyone to understand Systems Thinking and build models. Instead
of worrying about calculus equations, users focus on the stocks and flows in a system, how
they interact, and what those interactions yield. And, as research publications and other
audiences increasingly require researchers and presenters to show their work, the isee Exchange™
has made it easier to post and share models.”
Research Question: How do oyster shells regenerate?
“Despite evidence to the contrary, marine biologists still believe that the regeneration of
oyster shell material is an extracellular activity,” says Mount. In fact, through years of
research and hypothesis testing, Mount discovered that the crystals that combine to form
oyster shells are generated inside the animal’s cells.
That research began at the College of Charleston when Mount was earning a Master’s degree.
“Lowell Seick, my mentor and thesis advisor, hypothesized that free amino acids secreted
from oyster tissue into the fluid-filled space between the animal’s body and shell participated
in nucleation,” says Mount. “Simply put, he thought the amino acids participated in calcium
binding with carbonate to create crystals needed for shell building. He tasked me with finding
those amino acids in the fluid.”
Through much trial and error and microscope-assisted searching, Mount did find free amino acids
in the fluid he extracted from the space between the oyster shell and body. He built a model to
demonstrate how the amino acids assisted the binding of calcium from outside the oyster’s cells
to carbonate inside the cell to create new crystals.
Mount and Seick were pleased with the discovery but were presented with a new question.
Dr. A.P. (Hap) Wheeler from Clemson University, a member of Mount’s thesis committee, asked
if nucleation was impacted by the salinity, or salt content, of the fluid. “I realized I
needed a way to run lots of experiments without running an actual experiment. That’s where
Stella came in,” says Mount.
Using a Stella model allowed Mount to vary concentrations of protein, saline, and other fluid
contents and look for nucleation. “It was a mathematical model and I used it to run about 70
experiments,” says Mount. “I was able to determine that the phosphoprotein didn’t nucleate
anything. Instead, it sticks to calcium carbonate crystals, which helps new crystals attach
to the existing shell.”
Now Mount understood the protein’s role in shell building, but where were the crystals coming from?
“I came to the conclusion that nucleation wasn’t extracellular,” says Mount. “It was happening
inside the cell. Unfortunately, with little to no research that concurs with mine, marine
biologists have largely ignored my findings.”
They also seem to be ignoring reality. Ocean acidity levels have been rising, which would negatively
impact extracellular nucleation. The environment outside the oyster’s body would be unfavorable.
On the contrary, oyster populations are doing well.
Teaching Question: How fast will a pandemic spread and how many people will be infected?
“Teaching students to use Systems Thinking, as opposed to giving them a dusty book, has real value,”
says Mount. “The model of professor as gatekeeper—students show up for a lecture, take notes, and
then regurgitate for the exam—is really antiquated. We need to teach students to think, run data,
and understand what’s happening.” Stella has been Mount’s classroom assistant for teaching students
how to think and gain insights.
In 2003, almost 20 years before COVID-19 exploded into a global pandemic, Mount and his students
modeled the Severe Acute Respiratory Syndrome (SARS) epidemic. The potentially catastrophic virus
first appeared in Hong Kong and, like COVID-19, was highly contagious and could be fatal.
Hong Kong notified the World Health Organization (WHO) of the SARS outbreak in March 2003. Cases
had been slowly building for several months and, as people travelled, other countries began
reporting infections. WHO issued a global alert, and the world feared a pandemic.
“At the time, my senior biology seminar students were studying plagues,” says Mount. “When
SARS hit the news, they became excited about modelling the outbreak to understand how fast
the virus could spread and the number of people it could infect.”
The class took a mathematical ecology population-level deterministic approach to model building.
Students focused on the number of susceptible people in a population and then applied the WHO’s
reported rate of infection. Then they applied a recovery rate to the infected population.
Deterministic models assume average rates—in the case of SARS, average rates of infection—
with no deviations across populations. Student SARS models considered infection rates across a
global population without factoring in social, cultural, political, or other factors that would
differentiate populations.
The class was divided into four-person groups that included a leader, a devil’s advocate who would
question assumptions, a reporter, and a graphics person. Students who were experienced Stella users
mentored peers who were new to modeling and the software. Each group built a model that simulated the
population movement across susceptible, exposed, infective, and recovery states, or SEIR model. Models
were driven by WHO data that reported the number of cases and mortalities every week.
WHO reports began in January and, by March, infection rates were growing. “The rate of infection or basic
reproduction rate (R0) for SARS was 2.7, meaning that every person who contracted SARS would, on average,
infect three others,” says Mount. “Any R0 over one indicates a pandemic. In calculating recovery, models
applied WHO data that indicated 17% of infected people died.
“When students first ran their models they thought, ‘Oh no! The whole world will get SARS!”
says Mount. In fact, as their models showed, SARS has a rapid onset, short peak, and rapid decline.
That was because SARS' effective reproduction number declined as the population recovered.” In contrast,
COVID-19 became a global crisis because while “COVID-19’s effective reproduction number also declined over
time, the virus’ rate of mutation is incredibly high, with each successive variant having a higher R0.”
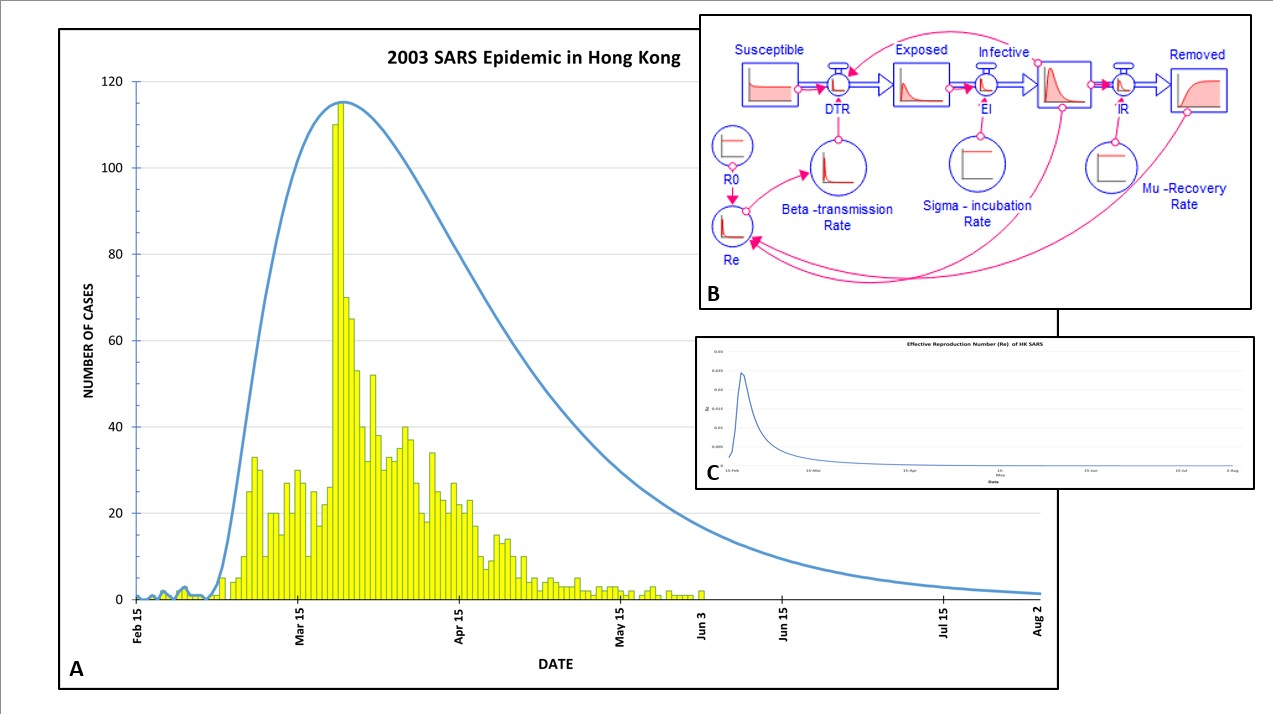 2003 SARS Epidemic in Hong Kong A. The outbreak (yellow) compared to Stella model (blue line) B.
Stella diagram showing the S-E-I-R epidemiological model C. Effective reproduction rate (Re) is dependent
on the number of recovered cases.
2003 SARS Epidemic in Hong Kong A. The outbreak (yellow) compared to Stella model (blue line) B.
Stella diagram showing the S-E-I-R epidemiological model C. Effective reproduction rate (Re) is dependent
on the number of recovered cases.
Product Innovation Question: Can an environmentally safe, biologic coating effectively replace
the toxic, copper-based paint currently used to keep marine vessels clean of barnacles and other biofoulers?
Retired from teaching, Mount is now using Stella to develop an environmentally safe marine coating.
From 2005 to 2018, he studied the cellular biology of barnacle biofouling supported by funding from
the Office of Naval Research (ONR). ONR was looking for an alternative to the toxic copper paints that
are currently used to keep vessel hulls free of barnacles. Now, Mount is translating that lab’s biofouling
deterrence research into marketable anti-fouling technology.
“The grail is a clean hull that doesn’t have to be scraped and painted for 10 years,” says Mount.
“We discovered that barnacles, oysters, bryozoans, and other biofoulers have specialized adrenergic
cellular receptors. When disrupted by a conopeptide antagonist, which originates from secretory cells
that line cone snail venom ducts, they won’t settle on a treated surface. The question was, could that
biological deterrent replace toxic, copper-based paint.”
“In one year, a 30’ fishing boat coated with copper-based paint will leach over two pounds of copper
into the ocean. Using a Stella model fitted to peer-reviewed data, we’ve been able to demonstrate a
positive impact of replacing copper-based paint with an environmentally responsible coating. Just one
gram of our biologic coating replaces 5.5 pounds of copper per gallon of antifouling paint.”
The modeled rate of copper release into the ocean is extremely precise, making it easy to predict toxicity
effects for over 78 known marine species. Mount is finishing his manuscript and preparing to upload his model
to the isee Exchange.
“The model will help the scientific and engineering communities communicate,” says Mount. “Accessible to anyone,
even someone with no Stella experience, it will help potential investors through their due diligence process,
provide a rationale for the resistant paint market to adopt a better solution, enable regulators and policy
makers to craft better legislation and guidelines, and give consumers the information they need.”
 Copper footprint of a 30-foot fishing boat. A. Published leaching data (blue) compared to Stella model
(red) B. Stella model for copper epoxy antifouling paint. The model is within a 0.5% agreement of the
actual leaching data at day 60 of the simulation.
Copper footprint of a 30-foot fishing boat. A. Published leaching data (blue) compared to Stella model
(red) B. Stella model for copper epoxy antifouling paint. The model is within a 0.5% agreement of the
actual leaching data at day 60 of the simulation.